This is the second part of our post on substitution and elimination where we will focus on elimination reactions. If you missed the first part of this series, you can click here to view the substitution reactions. One thing to keep in mind when you hear the term "elimination" in organic chemistry is that you should immediately think of double bond formation. So, with the study of elimination reactions, we will be discussing double bond formation.
Just like with substitution reactions, we also have two types of elimination reactions to discuss, elimination unimolecular (E1), and elimination bimolecular (E2). In the E1 reaction, the mechanism takes place in two steps, just like its SN1 counterpart; the leaving group leaves and the "nucleophile" now acting as a base, causes an elimination reaction to occur forming an alkene. In the E2 reaction, the mechanism is concerted (all in a single step) just like its SN2 counterpart. Before we look at how these reactions happen and break down each one, let's talk about alkene formation.
When forming alkenes, we typically use two sets of rules to govern the type of alkenes; the Zatisev rule and the Hoffmann rule or the Hofmann product. The Zaitsev rule states that "in the elimination of HX from an alkane, the alkene that is the most substituted is formed." The Hofmann product is the alkene that is least substituted. The majority of the time, with normal bases, the Zaitsev rule predominates. However, when using strong bulky bases, the Hofmann product is formed. An example of a bulky base would be potassium t-butoxide.

We can also have variability in the geometry of the alkenes that are formed. Most of the time, the trans alkene is formed predominately, since it is more stable. However, there could also be some of the cis alkene that is formed as a minor product.
Now, let's look into the actual reactions that are taking place, we will start with the E2 reaction first. As we discussed earlier, the E2 reaction is a concerted reaction in which the rate depends on both the substrate and base concentrations. Thus, we have a similar rate equation to SN1 reactions. The rate equation for E2 reactions can be defined by rate = k[substrate][base]. This means that both the substrate and the base are equally involved in the reaction. So, if you double the amount of substrate, the rate doubles. If you double the amount of base, the rate doubles. There is also a geometrical requirement of the substrate, however. The proton being abstracted by the base and the leaving group must be in an anti-periplanar geometry. Wait, what? This is a fancy way of saying that the two need to be overlapping in the same plane.

In an E2 reaction, a strong base works the best (remember, it is part of the rate equation). However, as stated above, we can increase the bulk of the base and cause eliminations to give the Hoffman product. An example of a strong bulky base would be potassium t-butoxide. Another aspect to consider is the bulkiness of the substrate as well. The more substituted the substrate, the higher the propensity for an elimination versus substitution with a strong base. Look below for an example of an E2 reaction.

The next type of elimination is the E1 reaction. Just like the SN1 reaction, the E1 reaction has a rate that is solely dependent on the substrate. Thus, the rate equation is rate = k[substrate]. Usually, a weak base is used in this reaction. The reaction is a two-step reaction that has a carbocation intermediate. Therefore, carbocation stability is a key factor in this reaction. The conditions for the E1 reaction are markedly similar to the conditions for the SN1 reaction, therefore mixtures are common. One way to increase the propensity for an E1 reaction is to add heat. Have a look below to see an example of an E1 reaction.

Notice in the above reaction that the more highly substituted alkene is formed. Also notice that the base here is also the solvent and it is very weak (not charged). This is common in both SN1 and E1 reactions so it's something you should look out for when determining the type of reaction.
Another aspect to watch out for when dealing with carbocation intermediates is the possibility of rearrangements. Sometimes a carbocation can rearrange to gain some stability. My rule of knowing when this happens is to go with the montage of "if it can, it will." Take a look at the reaction below and guess the products.

Let's first analyze the reaction to determine what type of reaction this is. First, my substrate is a secondary alkyl halide (this doesn't really tell us very much). Next, we can move to our nucleophile/base. Is this a strong nucleophile/base? This is just methanol so it is a weak nucleophile/base (not charged). Solvent? Here, the nucleophile/base is our solvent (very common for SN1 and E1 reactions) so we should be leaning towards SN1or E1. Is there any heat added? No, so for this reaction, we should be thinking SN1. Therefore, the product should be something like below.

But...this is not the major product we actually see. The product is different because here, we have the potential for a carbocation rearrangement. The carbocation generated in this reaction is initially a secondary carbocation. However, there is a methyl group that is adjacent to the carbocation that is formed. The methyl group can "shift" with its electrons over to the secondary carbocation. By moving, the newly formed carbocation becomes tertiary and is more stable. Take a look below to see how this happens.

A final thought on carbocation rearrangements: Remember, rearrangements can only happen if there is a hydrogen or an alkyl group next to the carbocation.
Now, let's sum things up and put it all together. Let's do a few problems and show how we can identify the type of reaction that is occurring.

We can attack this problem using a methodical approach. First, we want to analyze the substrate. Here, we can see that the substrate is a primary alkyl halide. Since it is primary, SN1and E1 are out. Next, we need to look at our nucleophile/base. Are there any charges that aren't listed? In this case, our nucleophile is sodium hydrosulfide and should have a positive charge on the sodium and a negative on the sulfur. Remember that things with charges are often left out and it is up to you to know that they are there! Here, we have a charged, strong nucleophile. This nucleophile therefore favors SN2. Finally, we can look at the solvent. The solvent is polar aprotic and therefore strongly favors an SN2 reaction.
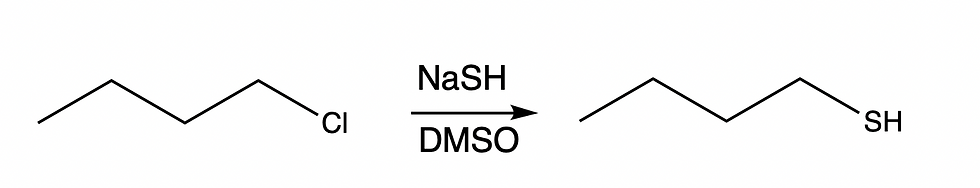
Now, let's look at another problem and use the same method to solve it. Remember, we are going to look at each piece separately until we have a consensus.

First, our substrate is a secondary alkyl halide so that doesn't help us really narrow down anything. So, we need to move forward and analyze the other pieces to make a decision. We can see that the solvent is the nucleophile/base here. Remember, when the solvent is the nucleophile/base, that is a strong indicator for SN1/E1. Also, the solvent here is a polar protic solvent so that strongly favors SN1/E1. There is no heat added so we should expect to see a SN1 reaction. If that is the case, then we know we are going to have a carbocation. Is there a way that the carbocation can rearrange? The initial carbocation is secondary but a hydride shift would make the new carbocation tertiary! Therefore, we have a SN1 reaction with a 1,2-hydride shift to give us our final product.

Hope these posts helped you in understanding substitution and elimination!

Comments